Research and Development | Oncology

Estrogen Receptor
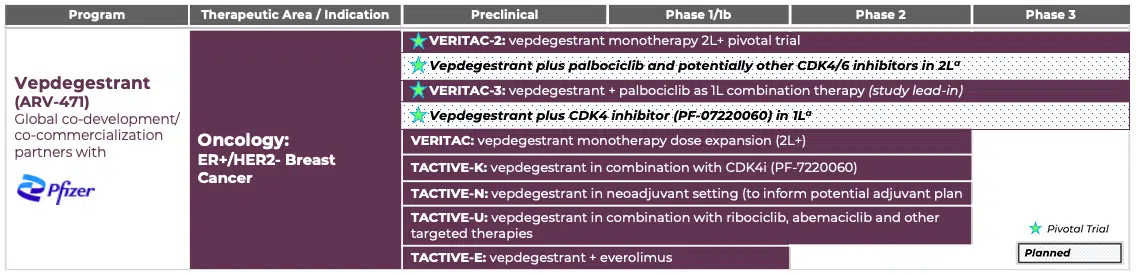
Vepdegestrant (ARV-471): a PROTAC® Estrogen Receptor Degrader for Breast Cancer
Vepdegestrant is currently under investigation. The safety and effectiveness of vepdegestrant have not yet been established.


About the Estrogen Receptor
What is estrogen receptor?
Estrogen receptor (ER) is a nuclear hormone receptor. The ER is part of a normal cellular pathway in which estrogen (a hormone) binds to the ER, leading to its movement to the cell’s nucleus and interactions with other signaling proteins. Breast cancers that test positive for ER are called ER-positive (or ER+) cancers.
How does ER relate to breast cancer?
Many breast cancers are known to be driven by estrogen receptor signaling. Women with metastatic ER+ breast cancer are often treated with hormone therapy (also referred to as endocrine therapy) , but in patients with aggressive disease, or whose disease continues to progress with a hormonal treatment regimen, additional hormonal therapy may be warranted. A significant unmet need remains for patients with ER+ breast cancer.
Why is ER an important target?
Breast cancer is the most common cancer and the second leading cause of cancer death in women in the United States. Approximately 80% of all newly diagnosed cases of breast cancer are ER+. ER degradation has been a long-standing therapeutic focus for Arvinas given the well-documented biology of ER signaling as a principal driver in breast cancer.
A Potential Novel Hormonal Therapy for ER+ Breast Cancer
ER is the primary driver of hormone receptor (HR) positive breast cancer, which is the most common breast cancer subtype. Endocrine therapy is a backbone of ER+ breast cancer treatment and is used as monotherapy or as combination therapy as a standard of care across treatment settings.
Preclinical Studies
In preclinical studies, vepdegestrant demonstrated up to 97% ER degradation in tumor cells, induced robust tumor shrinkage when dosed as a single agent in multiple ER-driven xenograft models, and showed increased anti-tumor activity compared to fulvestrant, both as a single agent and in combination with CDK4/6 inhibitors. Vepdegestrant is a PROTAC® protein degrader that directly recruits the ubiquitin proteasome system to degrade the ER.
Learn More About Our Research
Access our most recent preclinical and clinical data on Vepdegestrant (ARV-471).
- Lumachi, Santeufemia, Basso. Current medical treatment of estrogen receptor-positive breast cancer. World J Biol Chem. 2015 August 26;6(3) 231-239.
- Flanagan et al. SABCS 2018
- The et al. Enhanced efficacy of vepdegestrant (ARV 471), a novel PROTAC® estrogen receptor degrader, in combination with targeted agents in ER+ breast cancer models. AACR 2023
- Hormone Therapy for Breast Cancer was originally published by the National Cancer Institute.