Research and Development

Neuroscience
Advancing the next generation of treatments for diseases of the central nervous system


Propelling Research for Treating Diseases of the Central Nervous System
Attacking the Problem Using Differentiated Pharmacology

Potential differentiation of the investigational PROTAC® platform:
Ability to cross the blood-brain barrier, which is a substantial challenge for monoclonal antibodies and genomic therapies.
Ability to enter cells of the CNS, which may allow for the degradation of pathogenic proteins (such as mutant or oligomeric tau) from the inside of the cell, thus preventing both intracellular dysfunction and “prion-like” spread of pathologic protein into the extracellular space and healthy neurons.
Ability to selectively degrade mutant forms of pathologic proteins and spare healthy copy of the protein (such as for mutant Huntingtin protein (mHTT)).
Degradation enables a PROTAC® protein degrader to disrupt the scaffolding function of a disease-causing target protein.
Iterative (often described as “catalytic”) activity, by which a single PROTAC® protein degrader molecule can induce the degradation of hundreds of copies of the disease-causing protein.
Directed degradation to appropriate tissues while avoiding others by cell- or tissue-restricted E3 ligases.
Oral dosing provides an additional route of administration for the targeted elimination of disease-causing proteins from the CNS.
Preclinical Studies
In preclinical studies to date, Arvinas has demonstrated the ability to create PROTAC® protein degraders that can be dosed either orally or parenterally and cross the blood-brain barrier. Arvinas believes our ongoing research on PROTAC® protein degraders represent a promising new approach to treating diseases linked to pathogenic tau, alpha-synuclein, and other important targets previously considered undruggable.
Arvinas is progressing four named programs (leucine-rich repeat kinase 2- LRRK2, mutant Huntingtin – mHTT, tau, and alpha-synuclein), and a number of undisclosed programs.
Optimizing LRRK2-Targeting PROTAC® Protein Degraders For Selectivity and to Cross the Blood-Brain Barrier Following Oral Administration
Arvinas has optimized selected LRRK2 PROTAC® protein degraders to show improved potency and degradation in the brain following a single oral administration in mice. In the left panel below, PROTAC-B (purple circles) has been optimized for improved LRRK2 potency in the cortex of the mouse brain compared to PROTAC-A (orange circles) following a single oral administration of different dose levels. In the panel on the right, following a single oral PROTAC® administration, the concentration of PROTAC® in the brain (in open circles- right Y-axis) induces durable 50% degradation of the LRRK2 protein (orange squares- left Y-axis) for two to three days. As the PROTAC® is cleared from the brain over time (to the lower limit of quantification (lloq)), LRRK2 protein in orange squares is resynthesized and restored to the baseline protein expression level. These data show that PROTAC® degrader molecules are eliminated from the brain and the LRRK2 protein is resynthesized within days, which is unlike genomic modalities where the target protein may be impacted for months or longer.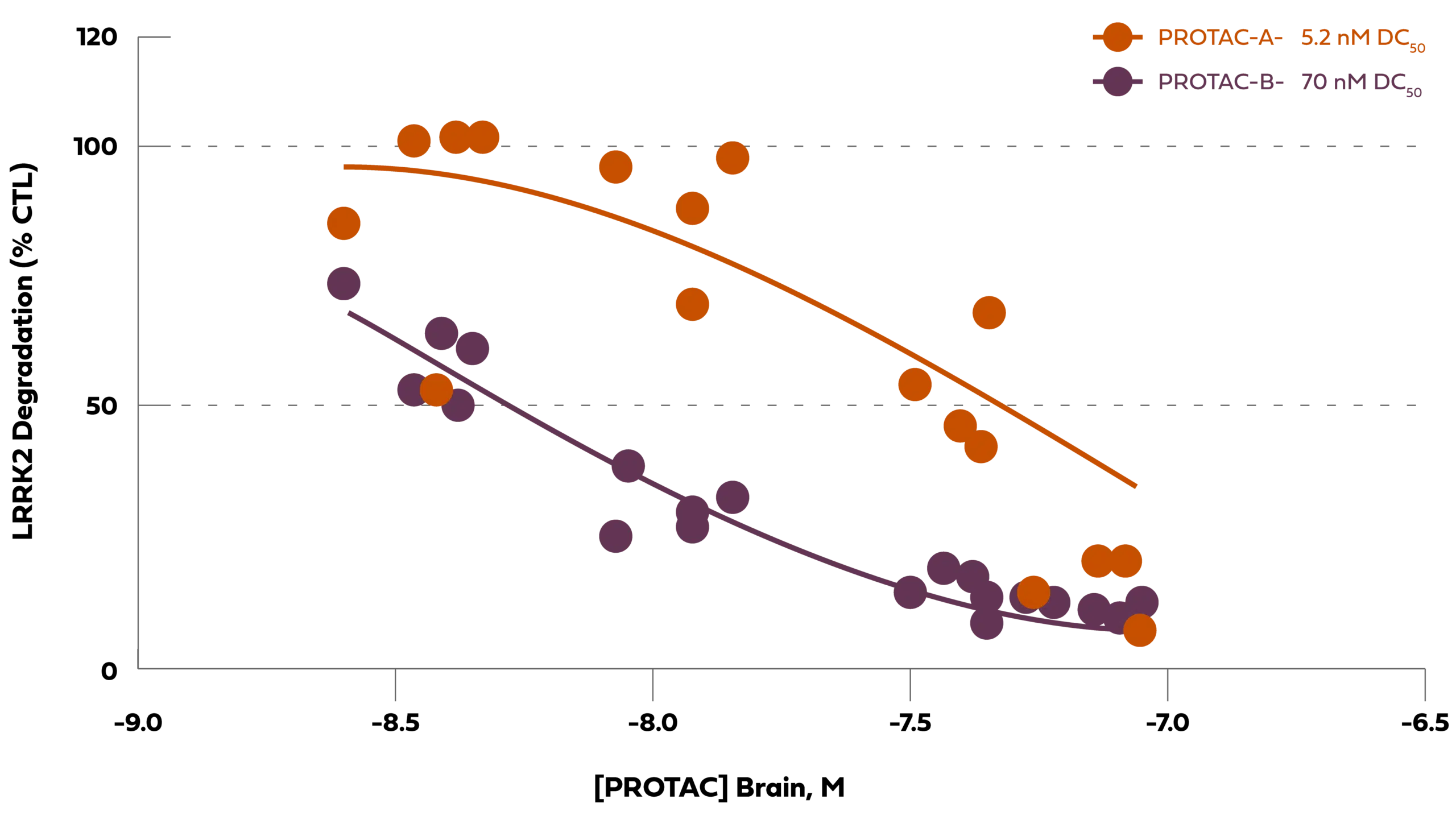
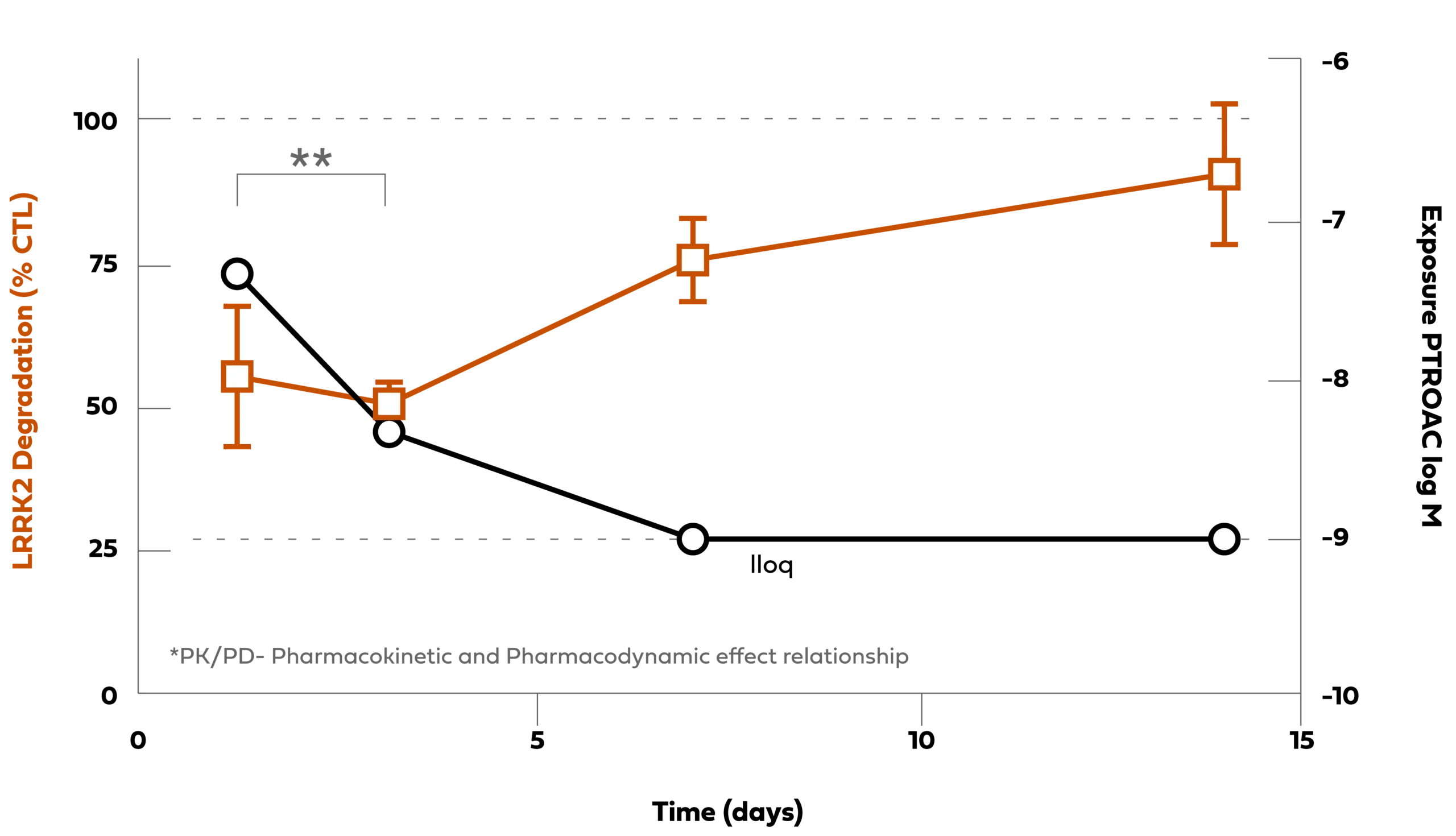
Chart Description:A single oral administration of our PROTAC® protein degrader rapidly degrades LRRK2 in brain (concentration-dependent and durable)
CTL:Vehicle Control; DC50:half-maximal degradation concentration; M: Molar
As shown below, our BBB-penetrant LRRK2 PROTAC® molecules have been shown to demonstrate very high selectivity for degradation of the LRRK2 protein (orange circle), compared to many other proteins measurable by mass spectroscopy (in purple circles) in the mouse brain cortex 24 hours following a single oral (PO) administration, and this selectivity was highly statistically significant (p > 10-17).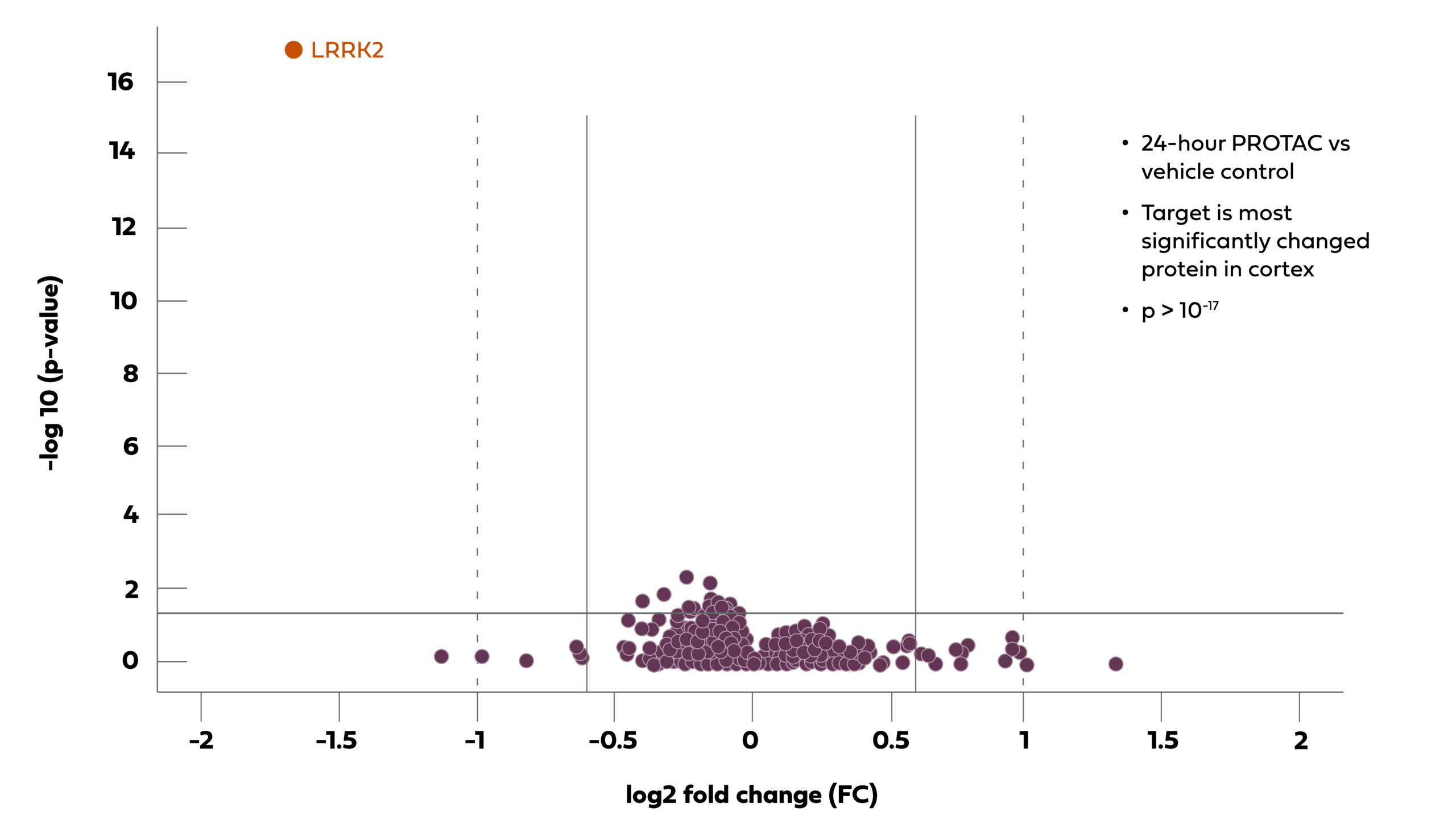
Chart Description: Arvinas’ oral LRRK2 PROTAC® protein degrader molecule is highly selective in the brain
Our BBB-penetrant oral PROTAC® molecules can be optimized to show degradation of the LRRK2 protein in the brain across species (see Keystone Presentation) and broadly biodistributed to deep anatomic regions of the primate brain. As shown in the figure below, following oral administration the LRRK2 PROTAC® -induced degradation of LRRK2 across the primate brain including the cortex, striatum, caudate (not shown), and cerebellum. These brain regions are important in neurodegenerative diseases and are difficult for biologic and genomic modalities to get to by intravenous or intrathecal dosing routes (respectively).Thus, further differentiating our BBB-penetrant PROTAC® technology as a modality that can be orally administered and can uniformly biodistribute across the brain.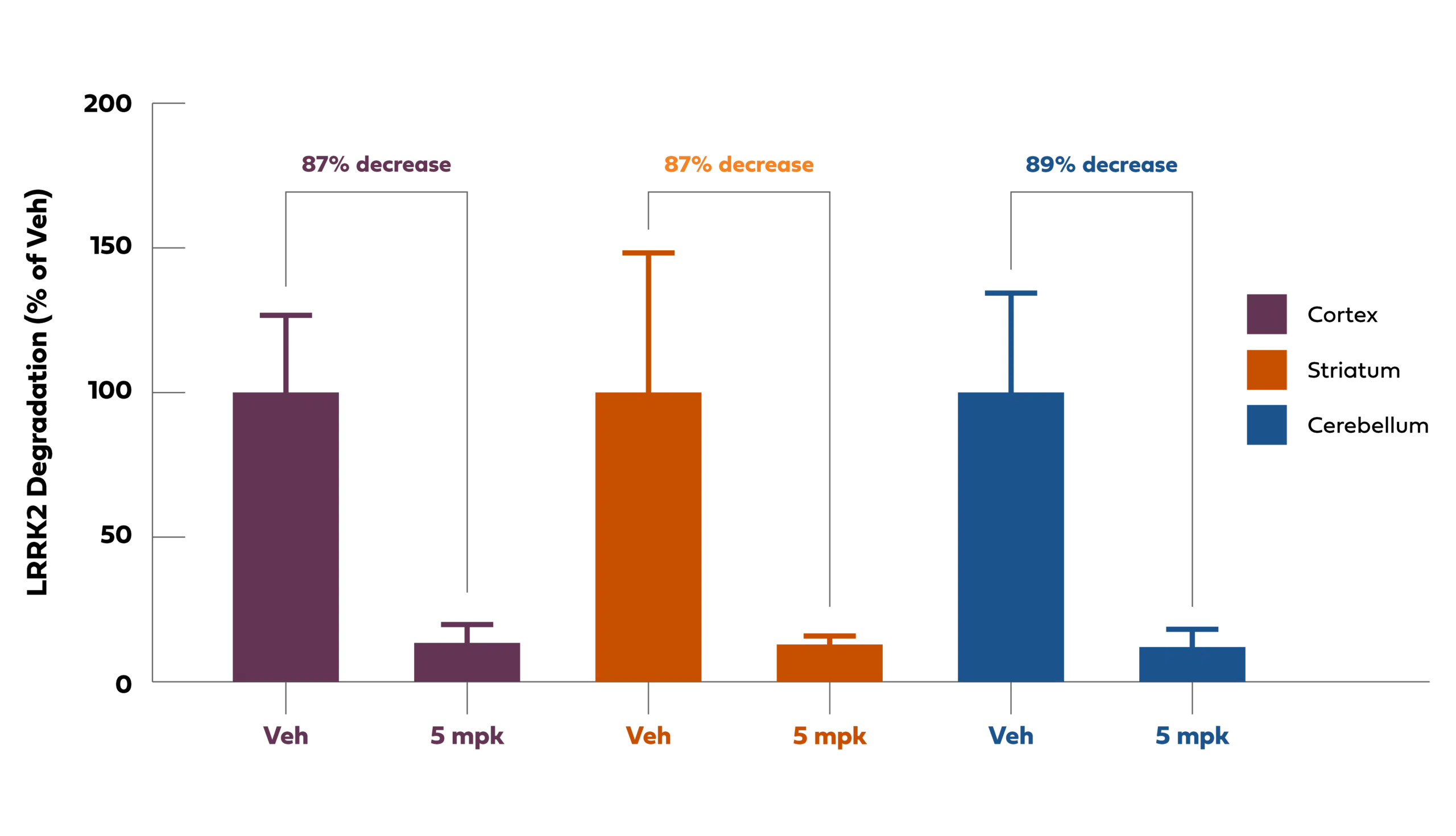
Chart Description: Oral PROTAC®-induced LRRK2 degradation with biodistribution to deep brain regions
Huntington’s disease is caused by a mutation in the huntingtin (HTT) gene. PROTAC® protein degradation has the potential to enable selective targeting of mutant HTT (mHTT) protein. We have identified novel ligands that, when incorporated into a PROTAC® protein degrader molecule, induce potent and selective degradation of mHTT (shown in the panel below, in purple). Protein degradation requires binding to both the target (competed by addition of mHTT ligand in pink circles) and E3 ligase (E3-dead PROTAC in orange circles). The PROTAC® molecule induces potent degradation of mHTT (in purple) while sparing the protein produced by the wild-type HTT allele (WT HTT shown in blue).
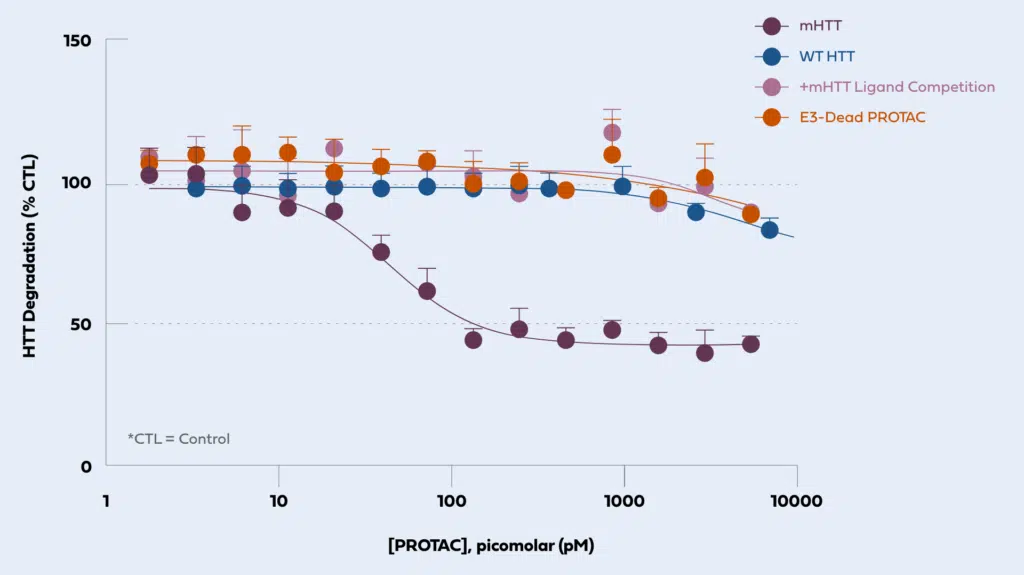
Chart Description: Huntington’s Disease: Ligand chemistry enables allele-selective PROTAC® degradation of mHTT
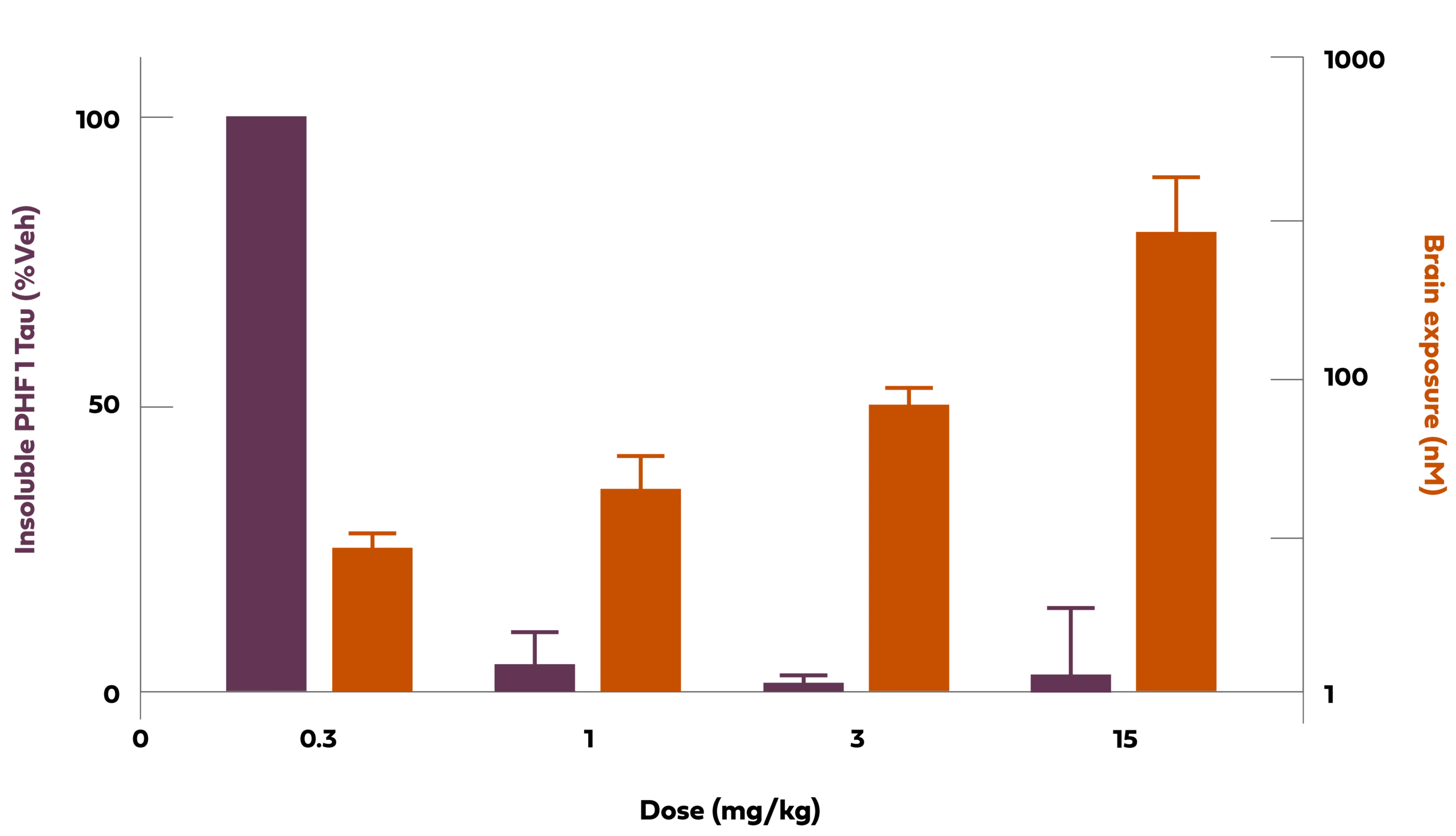 Chart Description: 1 Tg2508 is a murine pathologic tau model (P301L).nM: nanomolar;mg/kg:Milligrams per kilogram;veh: vehicle; PHF1 is an antibody direct against phosphorylated Ser396 and Ser404.
Chart Description: 1 Tg2508 is a murine pathologic tau model (P301L).nM: nanomolar;mg/kg:Milligrams per kilogram;veh: vehicle; PHF1 is an antibody direct against phosphorylated Ser396 and Ser404.Alpha-synuclein (α-syn) is an abundant neuronal protein that localizes predominantly to presynaptic terminals within the brain. While the precise normal function of this protein is not fully understood, the pathologic aggregation of α-syn is implicated in synucleinopathies. The most common of these diseases is Parkinson’s.
Arvinas is working on approaches to degrade α-syn and remove this protein from neurons that lead to pathology in diseases such as Parkinson’s Disease, multiple system atrophy, and Lewy body dementia.
View our latest neuroscience-focused scientific presentation:
The agents listed above are currently under investigation. Their safety and effectiveness have not yet been established.
- Cacace, A. (2019, October 24). Discovery of Brain Penetrant PROTAC Degrader Molecules That Target Pathologic Tau and alpha-Synuclein Protein Species [1-22]. Presented at: Targeted Protein Degradation Summit. Boston, MA. https://s3.us-east-1.amazonaws.com/arvinas-assets.investeddigital.com/scientific-publications/Cacace_TPD-10-24-2019_final.pdf
- Pardridge, W. M. (2020). Blood-brain barrier and delivery of protein and gene therapeutics to brain. Frontiers in Aging Neuroscience, 11. https://doi.org/10.3389/fnagi.2019.00373
- Mazur, C., Powers, B., Zasadny, K., Sullivan, J. M., Dimant, H., Kamme, F., Hesterman, J., Matson, J., Oestergaard, M., Seaman, M., Holt, R. W., Qutaish, M., Polyak, I., Coelho, R., Gottumukkala, V., Gaut, C. M., Berridge, M., Albargothy, N. J., Kelly, L., Carare, R. O., … Verma, A. (2019). Brain pharmacology of intrathecal antisense oligonucleotides revealed through multimodal imaging. JCI insight, 4(20), e129240. https://doi.org/10.1172/jci.insight.129240
- Kouhi, A., Pachipulusu, V., Kapenstein, T., Hu, P., Epstein, A. L., & Khawli, L. A. (2021). Brain Disposition of Antibody-Based Therapeutics: Dogma, Approaches and Perspectives. International journal of molecular sciences, 22(12), 6442. https://doi.org/10.3390/ijms22126442
- Silva M. C., Ferguson F. M., Cai Q., Donovan K. A., Nandi G., Patnaik D., et al. (2019). Targeted degradation of aberrant tau in frontotemporal dementia patient-derived neuronal cell models. Elife 8, e45457. 10.7554/eLife.45457. https://pubmed.ncbi.nlm.nih.gov/30907729/
- Silva, M. C., Nandi, G., Donovan, K. A., Cai, Q., Berry, B. C., Nowak, R. P., Fischer, E. S., Gray, N. S., Ferguson, F. M., & Haggarty, S. J. (2022). Discovery and optimization of tau targeted protein Degraders enabled by patient induced pluripotent stem cells-derived neuronal models of Tauopathy. Frontiers in Cellular Neuroscience, 16. https://doi.org/10.3389/fncel.2022.801179
- Bond M.J. and Crews, C.M. (2021). PROTACs come of age: entering the third decade of targeted protein degradation. RSC Chem. Biol. https://pubs.rsc.org/en/content/articlepdf/2021/cb/d1cb00011j
- Bekes M., Langley, D. R., and Crews, C.M. (2022). PROTAC targeted Protein Degraders: the past is prologue. Nat. Rev. Drug Disc. https://www.nature.com/articles/s41573-021-00371-6
- Cacace, A. (2021, July 14). PROTAC Discovery Engine: Harnessing power of oral blood brain barrier penetrant degraders and new E3 ligases (1-22). Presented at Protein Society Meeting. Virtual. https://s3.us-east-1.amazonaws.com/arvinas-assets.investeddigital.com/scientific-publications/Angela-Cacace-Protein-Society-July-2021_2021-08-06-014908_ebim.pdf
- Cacace, A.M., Meredith, J., Bryce, D., Sparks, S., Kimmel., L., Kelly, K., Gregory, J., Matchett, M., Nickischer, D., Hendricson, A., Naumann, G., Kyne, R., Wilson., J., Corradi, J., Soto, L., Jeong, Y., Pizzano, J., Cadelina, G., Revell, D., Snyder, L., Berlin, M. (2022, November 12-16). Presented at Society for Neuroscience. San Diego, CA. Clearance of Pathologic Proteins in Neurodegeneration by oral PROTAC® Degrader Molecules.
- Burslem, G. M., Smith, B. E., Lai, A. C., Jaime-Figueroa, S., McQuaid, D. C., Bondeson, D. P., Toure, M., Dong, H., Qian, Y., Wang, J., Crew, A. P., Hines, J., & Crews, C. M. (2018). The Advantages of Targeted Protein Degradation Over Inhibition: An RTK Case Study. Cell chemical biology, 25(1), 67–77.e3. https://doi.org/10.1016/j.chembiol.2017.09.009 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5831399/#__ffn_sectitle
- Wang, X., Negrou, E., Maloney, M. T., Bondar, V. V., Andrews, S. V., Montalban, M., Llapashtica, C., Maciuca, R., Nguyen, H., Solanoy, H., Arguello, A., Przybyla, L., Moerke, N. J., Huntwork-Rodriguez, S., & Henry, A. G. (2021). Understanding LRRK2 kinase activity in preclinical models and human subjects through quantitative analysis of LRRK2 and pT73 Rab10. Scientific reports, 11(1), 12900. https://doi.org/10.1038/s41598-021-91943-4
- Zhao, Y., & Dzamko, N. (2019). Recent Developments in LRRK2-Targeted Therapy for Parkinson’s Disease. Drugs, 79(10), 1037–1051. https://doi.org/10.1007/s40265-019-01139-4
- Henderson, M. X., Sengupta, M., McGeary, I., Zhang, B., Olufemi, M. F., Brown, H., Trojanowski, J. Q., & Lee, V. M. Y. (2019). LRRK2 inhibition does not impart protection from α-synuclein pathology and neuron death in non-transgenic mice. Acta neuropathologica communications, 7(1), 28. https://doi.org/10.1186/s40478-019-0679-5
- Berwick, D.C., Heaton, G.R., Azeggagh, S. et al. (2019). LRRK2 Biology from structure to dysfunction: research progresses, but the themes remain the same. Mol Neurodegeneration 14, 49 https://doi.org/10.1186/s13024-019-0344-2
- Cacace, A. (2019, October 24). Discovery of Brain Penetrant PROTAC Degrader Molecules That Target Pathologic Tau and alpha-Synuclein Protein Species [1-22]. Presented at: Targeted Protein Degradation Summit. Boston, MA. https://s3.us-east-1.amazonaws.com/arvinas-assets.investeddigital.com/scientific-publications/Cacace_TPD-10-24-2019_final.pdf
- Change, C.-W., Shao, S., Mucke, L. (2021). Tau: enabler of diverse brain disorders and target of rapidly evolving therapeutic strategies. Science. 371(6532). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8118650/pdf/nihms-1682624.pdf
- Rideout, H., Greggio, E., Kortholt, A., Nichols, R. J. (2022). Editorial: LRRK2—Fifteen Years From Cloning to the Clinic. Frontiers in Neuroscience. https://www.frontiersin.org/articles/10.3389/fnins.2022.880914
- Rocha, E.M., Keeney, M.T., Di Maio, R., De Miranda, B.R., Greenamyre, J.T. (2022). LRRK2 and idiopathic Parkinson’s disease. Trends in Neuroscience. 45(3), P224-236. https://www.cell.com/action/showPdf?pii=S0166-2236%2821%2900250-2
- Langston, R.G., Beilina, A., Reed, X., Kaganovich, A., Singleton, A.B., Blauwendraat, C., Gibbs, J.R., Cookson, M.R. (2022). Association of a common genetic variant with Parkinson’s disease is mediated by microglia. Science Translational Medicine. 14 (655). https://www.science.org/doi/10.1126/scitranslmed.abp8869
- Tabrizi SJ, Estevez-Fraga C, van Roon-Mom WMC, Flower MD, Scahill RI, Wild EJ, Muñoz-Sanjuan I, Sampaio C, Rosser AE, Leavitt BR. (2022) Potential disease-modifying therapies for Huntington’s disease: lessons learned and future opportunities. Lancet Neurol. (7):645-658. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7613206/pdf/EMS146360.pdf.
- Goedert M, Jakes R, Spillantini MG. The Synucleinopathies: Twenty Years On. (2017) J Parkinsons Dis. 7(s1):S51-S69. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5345650/pdf/jpd-7-jpd179005.pdf